Group 7 element
| Group 7 in the periodic table | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| |||||||||
| ↓ Period | |||||||||
| 4 | Manganese (Mn) 25 Transition metal | ||||||||
| 5 | Technetium (Tc) 43 Transition metal | ||||||||
| 6 | Rhenium (Re) 75 Transition metal | ||||||||
| 7 | Bohrium (Bh) 107 Transition metal | ||||||||
|
Legend
| |||||||||
Group 7, numbered by IUPAC nomenclature, is a group of elements in the periodic table. It contains manganese (Mn), technetium (Tc), rhenium (Re) and bohrium (Bh). This group lies in the d-block of the periodic table, and are hence transition metals. This group is sometimes called the manganese group or manganese family after its lightest member; however, the group itself has not acquired a trivial name because it belongs to the broader grouping of the transition metals.
The group 7 elements tend to have a major group oxidation state (+7), although this trend is markedly less coherent than the previous groups. Like other groups, the members of this family show patterns in their electron configurations, especially the outermost shells resulting in trends in chemical behavior. In nature, manganese is a fairly common element, whereas rhenium is rare, technetium only occurs in trace quantities, and bohrium is entirely synthetic.
Physical properties
[edit]The trends in group 7 follow, although less noticeably, those of the other early d-block groups and reflect the addition of a filled f-shell into the core in passing from the fifth to the sixth period. All group 7 elements crystallize in the hexagonal close packed (hcp) structure except manganese, which crystallizes in the body centered cubic (bcc) structure. Bohrium is also expected to crystallize in the hcp structure.[1]
The table below is a summary of the key physical properties of the group 7 elements. The question-marked value is predicted.[2]
| Name | Mn, manganese | Tc, technetium | Re, rhenium | Bh, bohrium |
|---|---|---|---|---|
| Melting point | 1519 K (1246 °C) | 2430 K (2157 °C) | 3459 K (3186 °C) | Unknown |
| Boiling point | 2334 K (2061 °C) | 4538 K (4265 °C) | 5903 K (5630 °C) | Unknown |
| Density | 7.21 g·cm−3 | 11 g·cm−3 | 21.02 g·cm−3 | 26-27 g·cm−3?[3][4] |
| Appearance | silvery metallic | silvery-gray | silvery-gray | Unknown |
| Atomic radius | 127 pm | 136 pm | 137 pm | 128 pm?[2] |
Chemical properties
[edit]Like other groups, the members of this family show patterns in its electron configuration, especially the outermost shells:
| Z | Element | No. of electrons/shell |
|---|---|---|
| 25 | manganese | 2, 8, 13, 2 |
| 43 | technetium | 2, 8, 18, 13, 2 |
| 75 | rhenium | 2, 8, 18, 32, 13, 2 |
| 107 | bohrium | 2, 8, 18, 32, 32, 13, 2 |
All the members of the group readily portray their group oxidation state of +7 and the state becomes more stable as the group is descended. Technetium also shows a stable +4 state whilst rhenium exhibits stable +4 and +3 states.
Bohrium may therefore also show these lower states as well. The higher +7 oxidation state is more likely to exist in oxyanions, such as perbohrate, BhO4−, analogous to the lighter permanganate, pertechnetate, and perrhenate. Nevertheless, bohrium(VII) is likely to be unstable in aqueous solution, and would probably be easily reduced to the more stable bohrium(IV).[5]
Compounds
[edit]Oxides
[edit]Manganese
[edit]
Manganese forms a variety of oxides: MnO, Mn3O4, Mn2O3, MnO2, MnO3 and Mn2O7. Manganese(II) oxide is an inorganic compound that forms green crystals. Like many monoxides, MnO adopts the rock salt structure, where cations and anions are both octahedrally coordinated. Also like many oxides, manganese(II) oxide is often nonstoichiometric: its composition can vary from MnO to MnO1.045.[6] Manganese(II,III) oxide is formed when any manganese oxide is heated in air above 1000 °C.[6] Considerable research has centred on producing nanocrystalline Mn3O4 and various syntheses that involve oxidation of MnII or reduction of MnVI.[7][8][9] Manganese(III) oxide is unlike many other transition metal oxides in that it does not adopt the corundum (Al2O3) structure.[6] Two forms are generally recognized, α-Mn2O3 and γ-Mn2O3,[10] although a high pressure form with the CaIrO3 structure has been reported too.[11] Manganese(IV) oxide is a blackish or brown solid occurs naturally as the mineral pyrolusite, which is the main ore of manganese and a component of manganese nodules. The principal use for MnO2 is for dry-cell batteries, such as the alkaline battery and the zinc–carbon battery.[6] Manganese(VII) oxide is dark green in its crystalline form. The liquid is green by reflected light and red by transmitted light.[12] It is soluble in carbon tetrachloride, and decomposes when in contact with water.
Technetium
[edit]
Technetium's main oxides are technetium(IV) oxide and technetium(VII) oxide. Technetium(IV) oxide was first produced in 1949 by electrolyzing a solution of ammonium pertechnetate under ammonium hydroxide. It has often been used to separate technetium from molybdenum and rhenium.[13][14][15] More efficient ways are the reduction of ammonium pertechnetate by zinc metal and hydrochloric acid, stannous chloride, hydrazine, hydroxylamine, ascorbic acid,[14] by the hydrolysis of potassium hexachlorotechnetate[16] or by the decomposition of ammonium pertechnetate at 700 °C under an inert atmosphere.[13][17][18] It reacts with oxygen to produce technetium(VII) oxide at 450 °C.
Technetium(VII) oxide can be prepared directly by the oxidation of technetium at 450-500 °C.[19] It is a rare example of a molecular binary metal oxide. Other examples are ruthenium(VIII) oxide and osmium(VIII) oxide. It adopts a centrosymmetric corner-shared bi-tetrahedral structure in which the terminal and bridging Tc−O bonds are 167pm and 184 pm respectively and the Tc−O−Tc angle is 180°.[20]
Rhenium
[edit]Rhenium's main oxides are rhenium(IV) oxide and rhenium(VII) oxide. Rhenium(IV) oxide is a gray to black crystalline solid that can be formed by comproportionation.[21] At high temperatures it undergoes disproportionation. It is a laboratory reagent that can be used as a catalyst. It adopts the rutile structure. It forms perrhenates with alkaline hydrogen peroxide and oxidizing acids.[22] In molten sodium hydroxide it forms sodium rhenate:[23]
- 2 NaOH + ReO2 → Na2ReO3 + H2O
Rhenium(VII) oxide can be formed when rhenium or its oxides or sulfides are oxidized a 500-700 °C in air.[24] It dissolves in water to give perrhenic acid. Heating Re2O7 gives rhenium(IV) oxide, signalled by the appearance of the dark blue coloration.[25] In its solid form, Re2O7 consists of alternating octahedral and tetrahedral Re centres. It is the raw material for all rhenium compounds, being the volatile fraction obtained upon roasting the host ore.[26]
Rhenium, in addition to the +4 and +7 oxidation states, also forms a trioxide. It can be formed by reducing rhenium(VII) oxide with carbon monoxide at 200 C or elemental rhenium at 4000 C.[27] It can also be reduced with dioxane.[28] It is a red solid with a metallic lustre that resembles copper in appearance, and is the only stable trioxide of the group 7 elements.
Halides
[edit]Manganese
[edit]Manganese can form compounds in the +2, +3 and +4 oxidation states. The manganese(II) compounds are often light pink solids. Like some other metal difluorides, MnF2 crystallizes in the rutile structure, which features octahedral Mn centers.[29] and it is used in the manufacture of special kinds of glass and lasers.[30] Scacchite is the natural, anhydrous form of manganese(II) chloride.[31] The only other currently known mineral systematized as manganese chloride is kempite - a representative of the atacamite group, a group of hydroxide-chlorides.[32] It can be used in place of palladium in the Stille reaction, which couples two carbon atoms using an organotin compound.[33] It can be used as a pink pigment or as a source of the manganese ion or iodide ion. It is often used in the lighting industry.[33]
Technetium
[edit]The following binary (containing only two elements) technetium halides are known: TcF6, TcF5, TcCl4, TcBr4, TcBr3, α-TcCl3, β-TcCl3, TcI3, α-TcCl2, and β-TcCl2. The oxidation states range from Tc(VI) to Tc(II). Technetium halides exhibit different structure types, such as molecular octahedral complexes, extended chains, layered sheets, and metal clusters arranged in a three-dimensional network.[34][35] These compounds are produced by combining the metal and halogen or by less direct reactions.
TcCl4 is obtained by chlorination of Tc metal or Tc2O7 Upon heating, TcCl4 gives the corresponding Tc(III) and Tc(II) chlorides.[35]
- TcCl4 → α-TcCl3 + 1/2 Cl2
- TcCl3 → β-TcCl2 + 1/2 Cl2
The structure of TcCl4 is composed of infinite zigzag chains of edge-sharing TcCl6 octahedra. It is isomorphous to transition metal tetrachlorides of zirconium, hafnium, and platinum.[35]

Two polymorphs of technetium trichloride exist, α- and β-TcCl3. The α polymorph is also denoted as Tc3Cl9. It adopts a confacial bioctahedral structure.[36] It is prepared by treating the chloro-acetate Tc2(O2CCH3)4Cl2 with HCl. Like Re3Cl9, the structure of the α-polymorph consists of triangles with short M-M distances. β-TcCl3 features octahedral Tc centers, which are organized in pairs, as seen also for molybdenum trichloride. TcBr3 does not adopt the structure of either trichloride phase. Instead it has the structure of molybdenum tribromide, consisting of chains of confacial octahedra with alternating short and long Tc—Tc contacts. TcI3 has the same structure as the high temperature phase of TiI3, featuring chains of confacial octahedra with equal Tc—Tc contacts.[35]
Several anionic technetium halides are known. The binary tetrahalides can be converted to the hexahalides [TcX6]2− (X = F, Cl, Br, I), which adopt octahedral molecular geometry.[37] More reduced halides form anionic clusters with Tc–Tc bonds. The situation is similar for the related elements of Mo, W, Re. These clusters have the nuclearity Tc4, Tc6, Tc8, and Tc13. The more stable Tc6 and Tc8 clusters have prism shapes where vertical pairs of Tc atoms are connected by triple bonds and the planar atoms by single bonds. Every technetium atom makes six bonds, and the remaining valence electrons can be saturated by one axial and two bridging ligand halogen atoms such as chlorine or bromine.[38]
Rhenium
[edit]The most common rhenium chlorides are ReCl6, ReCl5, ReCl4, and ReCl3.[6] The structures of these compounds often feature extensive Re-Re bonding, which is characteristic of this metal in oxidation states lower than VII. Salts of [Re2Cl8]2− feature a quadruple metal-metal bond. Although the highest rhenium chloride features Re(VI), fluorine gives the d0 Re(VII) derivative rhenium heptafluoride. Bromides and iodides of rhenium are also well known.
Like tungsten and molybdenum, with which it shares chemical similarities, rhenium forms a variety of oxyhalides. The oxychlorides are most common, and include ReOCl4, ReOCl3.
Organometallic compounds
[edit]Manganese
[edit]Organomanganese compounds were first reported in 1937 by Gilman and Bailee who described the reaction of phenyllithium and manganese(II) iodide to form phenylmanganese iodide (PhMnI) and diphenylmanganese (Ph2Mn).[39]
Following this precedent, other organomanganese halides can be obtained by alkylation of manganese(II) chloride, manganese(II) bromide, and manganese(II) iodide. Manganese iodide is attractive because the anhydrous compound can be prepared in situ from manganese and iodine in ether. Typical alkylating agents are organolithium or organomagnesium compounds.
The chemistry of organometallic compounds of Mn(II) are unusual among the transition metals due to the high ionic character of the Mn(II)-C bond.[40] The reactivity of organomanganese compounds can be compared to that of organomagnesium and organozinc compounds. The electronegativity of Mn (1.55) is comparable to that of Mg (1.31) and Zn (1.65), making the carbon atom (EN = 2.55) nucleophilic. The reduction potential of Mn is also intermediate between Mg and Zn.
Technetium
[edit]
Technetium forms a variety of coordination complexes with organic ligands. Many have been well-investigated because of their relevance to nuclear medicine.[41]
Technetium forms a variety of compounds with Tc–C bonds, i.e. organotechnetium complexes. Prominent members of this class are complexes with CO, arene, and cyclopentadienyl ligands.[42] The binary carbonyl Tc2(CO)10 is a white volatile solid.[43] In this molecule, two technetium atoms are bound to each other; each atom is surrounded by octahedra of five carbonyl ligands. The bond length between technetium atoms, 303 pm,[44][45] is significantly larger than the distance between two atoms in metallic technetium (272 pm). Similar carbonyls are formed by technetium's congeners, manganese and rhenium.[46] Interest in organotechnetium compounds has also been motivated by applications in nuclear medicine.[42] Unusual for other metal carbonyls, Tc forms aquo-carbonyl complexes, prominent being [Tc(CO)3(H2O)3]+.[42]
Rhenium
[edit]Dirhenium decacarbonyl is the most common entry to organorhenium chemistry. Its reduction with sodium amalgam gives Na[Re(CO)5] with rhenium in the formal oxidation state −1.[47] Dirhenium decacarbonyl can be oxidised with bromine to bromopentacarbonylrhenium(I):[48]
- Re2(CO)10 + Br2 → 2 Re(CO)5Br
Reduction of this pentacarbonyl with zinc and acetic acid gives pentacarbonylhydridorhenium:[49]
- Re(CO)5Br + Zn + HOAc → Re(CO)5H + ZnBr(OAc)
Methylrhenium trioxide ("MTO"), CH3ReO3 is a volatile, colourless solid has been used as a catalyst in some laboratory experiments. It can be prepared by many routes, a typical method is the reaction of Re2O7 and tetramethyltin:
- Re2O7 + (CH3)4Sn → CH3ReO3 + (CH3)3SnOReO3
Analogous alkyl and aryl derivatives are known. MTO catalyses for the oxidations with hydrogen peroxide. Terminal alkynes yield the corresponding acid or ester, internal alkynes yield diketones, and alkenes give epoxides. MTO also catalyses the conversion of aldehydes and diazoalkanes into an alkene.[50]
Polyoxometalates
[edit]The polyoxotechnetate (polyoxometalate of technetium) contains both Tc(V) and Tc(VII) in ratio 4: 16 and is obtained as the hydronium salt [H7O3]4[Tc20O68]·4H2O by concentrating an HTcO4 solution.[51] The first empirically isolated polyoxorhenate was the white [Re4O15]2− and contained Re(VII) in both octahedral and tetrahedral coordination.[52]
History
[edit]Manganese
[edit]Manganese dioxide, which is abundant in nature, has long been used as a pigment. The cave paintings in Gargas that are 30,000 to 24,000 years old are made from the mineral form of MnO2 pigments.[53] Manganese compounds were used by Egyptian and Roman glassmakers, either to add to, or remove, color from glass.[54] Use as "glassmakers soap" continued through the Middle Ages until modern times and is evident in 14th-century glass from Venice.[55]
Technetium and rhenium
[edit]Rhenium (Latin: Rhenus meaning: "Rhine")[56] was the last-discovered of the elements that have a stable isotope (other new elements discovered in nature since then, such as francium, are radioactive).[57] The existence of a yet-undiscovered element at this position in the periodic table had been first predicted by Dmitri Mendeleev. Other calculated information was obtained by Henry Moseley in 1914.[58] In 1908, Japanese chemist Masataka Ogawa announced that he had discovered the 43rd element and named it nipponium (Np) after Japan (Nippon in Japanese). In fact, what he had was rhenium (element 75), not technetium.[59][60] The symbol Np was later used for the element neptunium, and the name "nihonium", also named after Japan, along with symbol Nh, was later used for element 113. Element 113 was also discovered by a team of Japanese scientists and was named in respectful homage to Ogawa's work.[61]
Rhenium was rediscovered by Walter Noddack, Ida Noddack, and Otto Berg in Germany. In 1925 they reported that they had detected the element in platinum ore and in the mineral columbite. They also found rhenium in gadolinite and molybdenite.[62] In 1928 they were able to extract 1 g of the element by processing 660 kg of molybdenite.[63] It was estimated in 1968 that 75% of the rhenium metal in the United States was used for research and the development of refractory metal alloys. It took several years from that point before the superalloys became widely used.[64][65]
The discovery of element 43 was finally confirmed in a 1937 experiment at the University of Palermo in Sicily by Carlo Perrier and Emilio Segrè.[66] In mid-1936, Segrè visited the United States, first Columbia University in New York and then the Lawrence Berkeley National Laboratory in California. He persuaded cyclotron inventor Ernest Lawrence to let him take back some discarded cyclotron parts that had become radioactive. Lawrence mailed him a molybdenum foil that had been part of the deflector in the cyclotron.[67]
Bohrium
[edit]Two groups claimed discovery of the element bohrium. Evidence of bohrium was first reported in 1976 by a Soviet research team led by Yuri Oganessian, in which targets of bismuth-209 and lead-208 were bombarded with accelerated nuclei of chromium-54 and manganese-55 respectively.[68] Two activities, one with a half-life of one to two milliseconds, and the other with an approximately five-second half-life, were seen. Since the ratio of the intensities of these two activities was constant throughout the experiment, it was proposed that the first was from the isotope bohrium-261 and that the second was from its daughter dubnium-257. Later, the dubnium isotope was corrected to dubnium-258, which indeed has a five-second half-life (dubnium-257 has a one-second half-life); however, the half-life observed for its parent is much shorter than the half-lives later observed in the definitive discovery of bohrium at Darmstadt in 1981. The IUPAC/IUPAP Transfermium Working Group (TWG) concluded that while dubnium-258 was probably seen in this experiment, the evidence for the production of its parent bohrium-262 was not convincing enough.[69]
In 1981, a German research team led by Peter Armbruster and Gottfried Münzenberg at the GSI Helmholtz Centre for Heavy Ion Research (GSI Helmholtzzentrum für Schwerionenforschung) in Darmstadt bombarded a target of bismuth-209 with accelerated nuclei of chromium-54 to produce five atoms of the isotope bohrium-262:[70]
This discovery was further substantiated by their detailed measurements of the alpha decay chain of the produced bohrium atoms to previously known isotopes of fermium and californium. The IUPAC/IUPAP Transfermium Working Group (TWG) recognised the GSI collaboration as official discoverers in their 1992 report.[69]
Occurrence and production
[edit]Manganese
[edit]Manganese comprises about 1000 ppm (0.1%) of the Earth's crust and is the 12th most abundant element.[71] Soil contains 7–9000 ppm of manganese with an average of 440 ppm.[71] The atmosphere contains 0.01 μg/m3.[71] Manganese occurs principally as pyrolusite (MnO2), braunite (Mn2+Mn3+6)(SiO12),[72] psilomelane (Ba,H2O)2Mn5O10, and to a lesser extent as rhodochrosite (MnCO3).

The most important manganese ore is pyrolusite (MnO2). Other economically important manganese ores usually show a close spatial relation to the iron ores, such as sphalerite.[74][75] Land-based resources are large but irregularly distributed. About 80% of the known world manganese resources are in South Africa; other important manganese deposits are in Ukraine, Australia, India, China, Gabon and Brazil.[73] According to 1978 estimate, the ocean floor has 500 billion tons of manganese nodules.[76] Attempts to find economically viable methods of harvesting manganese nodules were abandoned in the 1970s.[77]
In South Africa, most identified deposits are located near Hotazel in the Northern Cape Province, with a 2011 estimate of 15 billion tons. In 2011 South Africa produced 3.4 million tons, topping all other nations.[78]
Manganese is mainly mined in South Africa, Australia, China, Gabon, Brazil, India, Kazakhstan, Ghana, Ukraine and Malaysia.[79]
For the production of ferromanganese, the manganese ore is mixed with iron ore and carbon, and then reduced either in a blast furnace or in an electric arc furnace.[80] The resulting ferromanganese has a manganese content of 30 to 80%.[74] Pure manganese used for the production of iron-free alloys is produced by leaching manganese ore with sulfuric acid and a subsequent electrowinning process.[81]
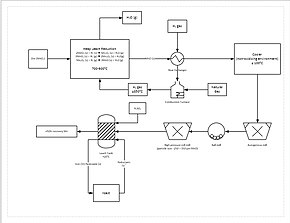
A more progressive extraction process involves directly reducing (a low grade) manganese ore in a heap leach. This is done by percolating natural gas through the bottom of the heap; the natural gas provides the heat (needs to be at least 850 °C) and the reducing agent (carbon monoxide). This reduces all of the manganese ore to manganese oxide (MnO), which is a leachable form. The ore then travels through a grinding circuit to reduce the particle size of the ore to between 150 and 250 μm, increasing the surface area to aid leaching. The ore is then added to a leach tank of sulfuric acid and ferrous iron (Fe2+) in a 1.6:1 ratio. The iron reacts with the manganese dioxide (MnO2) to form iron(III) oxide-hydroxide (FeO(OH)) and elemental manganese (Mn):
This process yields approximately 92% recovery of the manganese. For further purification, the manganese can then be sent to an electrowinning facility.[82]
In 1972 the CIA's Project Azorian, through billionaire Howard Hughes, commissioned the ship Hughes Glomar Explorer with the cover story of harvesting manganese nodules from the sea floor.[83] That triggered a rush of activity to collect manganese nodules, which was not actually practical. The real mission of Hughes Glomar Explorer was to raise a sunken Soviet submarine, the K-129, with the goal of retrieving Soviet code books.[84]
An abundant resource of manganese in the form of Mn nodules found on the ocean floor.[85][86] These nodules, which are composed of 29% manganese,[87] are located along the ocean floor and the potential impact of mining these nodules is being researched. Physical, chemical, and biological environmental impacts can occur due to this nodule mining disturbing the seafloor and causing sediment plumes to form. This suspension includes metals and inorganic nutrients, which can lead to contamination of the near-bottom waters from dissolved toxic compounds. Mn nodules are also the grazing grounds, living space, and protection for endo- and epifaunal systems. When theses nodules are removed, these systems are directly affected. Overall, this can cause species to leave the area or completely die off.[88] Prior to the commencement of the mining itself, research is being conducted by United Nations affiliated bodies and state-sponsored companies in an attempt to fully understand environmental impacts in the hopes of mitigating these impacts.[89]
Technetium
[edit]Technetium was created by bombarding molybdenum atoms with deuterons that had been accelerated by a device called a cyclotron. Technetium occurs naturally in the Earth's crust in minute concentrations of about 0.003 parts per trillion. Technetium is so rare because the half-lives of 97Tc and 98Tc are only 4.2 million years. More than a thousand of such periods have passed since the formation of the Earth, so the probability of survival of even one atom of primordial technetium is effectively zero. However, small amounts exist as spontaneous fission products in uranium ores. A kilogram of uranium contains an estimated 1 nanogram (10−9 g) equivalent to ten trillion atoms of technetium.[90][91][92] Some red giant stars with the spectral types S-, M-, and N contain a spectral absorption line indicating the presence of technetium.[93][94] These red giants are known informally as technetium stars.
Rhenium
[edit]
Rhenium is one of the rarest elements in Earth's crust with an average concentration of 1 ppb;[6][95] other sources quote the number of 0.5 ppb making it the 77th most abundant element in Earth's crust.[96] Rhenium is probably not found free in nature (its possible natural occurrence is uncertain), but occurs in amounts up to 0.2%[6] in the mineral molybdenite (which is primarily molybdenum disulfide), the major commercial source, although single molybdenite samples with up to 1.88% have been found.[97] Chile has the world's largest rhenium reserves, part of the copper ore deposits, and was the leading producer as of 2005.[98] It was only recently that the first rhenium mineral was found and described (in 1994), a rhenium sulfide mineral (ReS2) condensing from a fumarole on Kudriavy volcano, Iturup island, in the Kuril Islands.[99] Kudriavy discharges up to 20–60 kg rhenium per year mostly in the form of rhenium disulfide.[100][101] Named rheniite, this rare mineral commands high prices among collectors.[102]

Most of the rhenium extracted comes from porphyry molybdenum deposits.[103] These ores typically contain 0.001% to 0.2% rhenium.[6] Roasting the ore volatilizes rhenium oxides.[97] Rhenium(VII) oxide and perrhenic acid readily dissolve in water; they are leached from flue dusts and gasses and extracted by precipitating with potassium or ammonium chloride as the perrhenate salts, and purified by recrystallization.[6] Total world production is between 40 and 50 tons/year; the main producers are in Chile, the United States, Peru, and Poland.[104] Recycling of used Pt-Re catalyst and special alloys allow the recovery of another 10 tons per year. Prices for the metal rose rapidly in early 2008, from $1000–$2000 per kg in 2003–2006 to over $10,000 in February 2008.[105][106] The metal form is prepared by reducing ammonium perrhenate with hydrogen at high temperatures:[25]
- 2 NH4ReO4 + 7 H2 → 2 Re + 8 H2O + 2 NH3
- There are technologies for the associated extraction of rhenium from productive solutions of underground leaching of uranium ores.[107]
Bohrium
[edit]Bohrium is a synthetic element that does not occur in nature. Very few atoms have been synthesized, and also due to its radioactivity, only limited research has been conducted. Bohrium is only produced in nuclear reactors and has never been isolated in pure form.
Applications
[edit]
The facial isomer of both rhenium and manganese 2,2'-bipyridyl tricarbonyl halide complexes have been extensively researched as catalysts for electrochemical carbon dioxide reduction due to their high selectivity and stability. They are commonly abbreviated as M(R-bpy)(CO)3X where M = Mn, Re; R-bpy = 4,4'-disubstituted 2,2'-bipyridine; and X = Cl, Br.
Manganese
[edit]The rarity of rhenium has shifted research toward the manganese version of these catalysts as a more sustainable alternative.[108] The first reports of catalytic activity of Mn(R-bpy)(CO)3Br towards CO2 reduction came from Chardon-Noblat and coworkers in 2011.[109] Compared to Re analogs, Mn(R-bpy)(CO)3Br shows catalytic activity at lower overpotentials.[110]
The catalytic mechanism for Mn(R-bpy)(CO)3X is complex and depends on the steric profile of the bipyridine ligand. When R is not bulky, the catalyst dimerizes to form [Mn(R-bpy)(CO)3]2 before forming the active species. When R is bulky, however, the complex forms the active species without dimerizing, reducing the overpotential of CO2 reduction by 200-300 mV. Unlike Re(R-bpy)(CO)3X, Mn(R-bpy)(CO)3X only reduces CO2 in the presence of an acid.[110]
Technetium
[edit]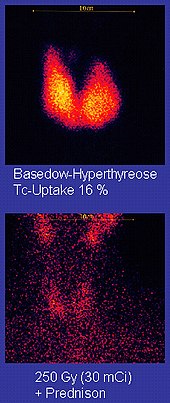
Technetium-99m ("m" indicates that this is a metastable nuclear isomer) is used in radioactive isotope medical tests. For example, Technetium-99m is a radioactive tracer that medical imaging equipment tracks in the human body.[90][111][112] It is well suited to the role because it emits readily detectable 140 keV gamma rays, and its half-life is 6.01 hours (meaning that about 94% of it decays to technetium-99 in 24 hours).[113] The chemistry of technetium allows it to be bound to a variety of biochemical compounds, each of which determines how it is metabolized and deposited in the body, and this single isotope can be used for a multitude of diagnostic tests. More than 50 common radiopharmaceuticals are based on technetium-99m for imaging and functional studies of the brain, heart muscle, thyroid, lungs, liver, gall bladder, kidneys, skeleton, blood, and tumors.[114] Technetium-99m is also used in radioimaging.[115]
The longer-lived isotope, technetium-95m with a half-life of 61 days, is used as a radioactive tracer to study the movement of technetium in the environment and in plant and animal systems.[116]
Technetium-99 decays almost entirely by beta decay, emitting beta particles with consistent low energies and no accompanying gamma rays. Moreover, its long half-life means that this emission decreases very slowly with time. It can also be extracted to a high chemical and isotopic purity from radioactive waste. For these reasons, it is a National Institute of Standards and Technology (NIST) standard beta emitter, and is used for equipment calibration.[117] Technetium-99 has also been proposed for optoelectronic devices and nanoscale nuclear batteries.[118]
Like rhenium and palladium, technetium can serve as a catalyst. In processes such as the dehydrogenation of isopropyl alcohol, it is a far more effective catalyst than either rhenium or palladium. However, its radioactivity is a major problem in safe catalytic applications.[119]
When steel is immersed in water, adding a small concentration (55 ppm) of potassium pertechnetate(VII) to the water protects the steel from corrosion, even if the temperature is raised to 250 °C (523 K).[120] For this reason, pertechnetate has been used as an anodic corrosion inhibitor for steel, although technetium's radioactivity poses problems that limit this application to self-contained systems.[121] While (for example) CrO2−
4 can also inhibit corrosion, it requires a concentration ten times as high. In one experiment, a specimen of carbon steel was kept in an aqueous solution of pertechnetate for 20 years and was still uncorroded.[120] The mechanism by which pertechnetate prevents corrosion is not well understood, but seems to involve the reversible formation of a thin surface layer (passivation). One theory holds that the pertechnetate reacts with the steel surface to form a layer of technetium dioxide which prevents further corrosion; the same effect explains how iron powder can be used to remove pertechnetate from water. The effect disappears rapidly if the concentration of pertechnetate falls below the minimum concentration or if too high a concentration of other ions is added.[122]
As noted, the radioactive nature of technetium (3 MBq/L at the concentrations required) makes this corrosion protection impractical in almost all situations. Nevertheless, corrosion protection by pertechnetate ions was proposed (but never adopted) for use in boiling water reactors.[122]
Rhenium
[edit]The catalytic activity of Re(bpy)(CO)3Cl for carbon dioxide reduction was first studied by Lehn et al.[123] and Meyer et al.[124] in 1984 and 1985, respectively. Re(R-bpy)(CO)3X complexes exclusively produce CO from CO2 reduction with Faradaic efficiencies of close to 100% even in solutions with high concentrations of water or Brønsted acids.[108]
The catalytic mechanism of Re(R-bpy)(CO)3X involves reduction of the complex twice and loss of the X ligand to generate a five-coordinate active species which binds CO2. These complexes will reduce CO2 both with and without an additional acid present; however, the presence of an acid increases catalytic activity.[108] The high selectivity of these complexes to CO2 reduction over the competing hydrogen evolution reaction has been shown by density functional theory studies to be related to the faster kinetics of CO2 binding compared to H+ binding.[110]
Bohrium
[edit]Bohrium is a synthetic element and is too radioactive to be used in anything.
Toxicity and precautions
[edit]Manganese compounds are less toxic than those of other widespread metals, such as nickel and copper.[125] However, exposure to manganese dusts and fumes should not exceed the ceiling value of 5 mg/m3 even for short periods because of its toxicity level.[126] Manganese poisoning has been linked to impaired motor skills and cognitive disorders.[127]
Technetium has low chemical toxicity. For example, no significant change in blood formula, body and organ weights, and food consumption could be detected for rats which ingested up to 15 μg of technetium-99 per gram of food for several weeks.[128] In the body, technetium quickly gets converted to the stable TcO−
4 ion, which is highly water-soluble and quickly excreted. The radiological toxicity of technetium (per unit of mass) is a function of compound, type of radiation for the isotope in question, and the isotope's half-life.[129] However, it is radioactive, so all isotopes must be handled carefully. The primary hazard when working with technetium is inhalation of dust; such radioactive contamination in the lungs can pose a significant cancer risk. For most work, careful handling in a fume hood is sufficient, and a glove box is not needed.[130]
Very little is known about the toxicity of rhenium and its compounds because they are used in very small amounts. Soluble salts, such as the rhenium halides or perrhenates, could be hazardous due to elements other than rhenium or due to rhenium itself.[131] Only a few compounds of rhenium have been tested for their acute toxicity; two examples are potassium perrhenate and rhenium trichloride, which were injected as a solution into rats. The perrhenate had an LD50 value of 2800 mg/kg after seven days (this is very low toxicity, similar to that of table salt) and the rhenium trichloride showed LD50 of 280 mg/kg.[132]
Biological role
[edit]Of the group 7 elements, only manganese has a role in the human body. It is an essential trace nutrient, with the body containing approximately 10 milligrams at any given time. It is present as a coenzyme in biological processes that include macronutrient metabolism, bone formation, and free radical defense systems. It is a critical component in dozens of proteins and enzymes.[133] The manganese in the human body is mainly concentrated in the bones, and the soft tissue remainder is concentrated in the liver and kidneys.[134] In the human brain, the manganese is bound to manganese metalloproteins, most notably glutamine synthetase in astrocytes.[135] Technetium, rhenium, and bohrium have no known biological roles. Technetium is, however, used in radioimaging.
References
[edit]- ^ Östlin, A.; Vitos, L. (2011). "First-principles calculation of the structural stability of 6d transition metals". Physical Review B. 84 (11): 113104. Bibcode:2011PhRvB..84k3104O. doi:10.1103/PhysRevB.84.113104.
- ^ a b Hoffman, Darleane C.; Lee, Diana M.; Pershina, Valeria (2006). "Transactinides and the future elements". In Morss; Edelstein, Norman M.; Fuger, Jean (eds.). The Chemistry of the Actinide and Transactinide Elements (3rd ed.). Dordrecht, The Netherlands: Springer Science+Business Media. ISBN 1-4020-3555-1.
- ^ Gyanchandani, Jyoti; Sikka, S. K. (10 May 2011). "Physical properties of the 6 d -series elements from density functional theory: Close similarity to lighter transition metals". Physical Review B. 83 (17): 172101. Bibcode:2011PhRvB..83q2101G. doi:10.1103/PhysRevB.83.172101.
- ^ Kratz; Lieser (2013). Nuclear and Radiochemistry: Fundamentals and Applications (3rd ed.). p. 631.
- ^ The chemistry of the actinide and transactinide elements (3rd ed.). Dordrecht: Springer. 2006. ISBN 978-1-4020-3555-5.
- ^ a b c d e f g h i Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ^ Hausmannite Mn3O4 nanorods: synthesis, characterization and magnetic properties Jin Du et al. Nanotechnology, (2006),17 4923-4928, doi:10.1088/0957-4484/17/19/024
- ^ One-step synthesis of Mn3O4 nanoparticles: Structural and magnetic study Vázquez-Olmos A., Redón R, Rodríguez-Gattorno G., Mata-Zamora M.E., Morales-Leal F, Fernández-Osorio A.L, Saniger J.M. Journal of Colloid and Interface Science, 291, 1, (2005), 175-180 doi:10.1016/j.jcis.2005.05.005
- ^ Sun, Xiaoming; Liu, Junfeng; Li, Yadong (2006-02-20). "Use of Carbonaceous Polysaccharide Microspheres as Templates for Fabricating Metal Oxide Hollow Spheres". Chemistry - A European Journal. 12 (7): 2039–2047. doi:10.1002/chem.200500660. ISSN 0947-6539. PMID 16374888.
- ^ Wells A.F. (1984) Structural Inorganic Chemistry 5th edition Oxford Science Publications ISBN 0-19-855370-6
- ^ High Pressure Phase transition in Mn2O3 to the CaIrO3-type Phase Santillan, J.; Shim, S. American Geophysical Union, Fall Meeting 2005, abstract #MR23B-0050
- ^ H. Lux (1963). "Manganese(VII) Oxide". In G. Brauer (ed.). Handbook of Preparative Inorganic Chemistry, 2nd Ed. Vol. 1. NY, NY: Academic Press. pp. 1459–1460.
- ^ a b A. G. Sharpe; H. J. Emeléus (1968). Advances in Inorganic Chemistry and Radiochemistry. Elsevier Science. p. 21. ISBN 9780080578606.
- ^ a b Edward Anders (1960). "THE RADIOCHEMISTRY OF TECHNETIUM". OSTI.GOV. U.S. Department of Energy Office of Scientific and Technical Information: 8. doi:10.2172/4073069. OSTI 4073069. Retrieved 4 November 2022.
- ^ L. B. Rogers (1949). "Electroseparation of Technetium from Rhenium and Molybdenum". Journal of the American Chemical Society. 71 (4): 1507–1508. doi:10.1021/ja01172a520.
- ^ C. M. Nelson; G. E. Boyd; Wm. T. Smith Jr. (1954). "Magnetochemistry of Technetium and Rhenium". Journal of the American Chemical Society. 76 (2). ACS Publications: 348–352. doi:10.1021/ja01631a009.
- ^ Bradley Covington Childs (2017). Volatile Technetium Oxides: Implications for Nuclear Waste Vitrification. UNLV Theses, Dissertations, Professional Papers, and Capstones (Thesis). doi:10.34917/10985836.
- ^ Edward Andrews (1959). "Technetium and Astatine Chemistry". Annual Review of Nuclear Science. 9. Annual Reviews: 203–220. Bibcode:1959ARNPS...9..203A. doi:10.1146/annurev.ns.09.120159.001223.
- ^ Herrell, A. Y.; Busey, R. H.; Gayer, K. H. (1977). Technetium(VII) Oxide, in Inorganic Syntheses. Vol. XVII. pp. 155–158. doi:10.1002/9780470132487.ch41. ISBN 0-07-044327-0.
- ^ Krebs, Bernt (1969). "Technetium(VII)-oxid: Ein Übergangsmetalloxid mit Molekülstruktur im festen Zustand". Angewandte Chemie. 81 (9): 328–329. Bibcode:1969AngCh..81..328K. doi:10.1002/ange.19690810905.
- ^ G. Glemser "Rhenium (IV) Oxide" Handbook of Preparative Inorganic Chemistry, 2nd Ed. Edited by G. Brauer, Academic Press, 1963, NY. Vol. 1. p. 1480.
- ^ "RHENIUM DIOXIDE - Manufacturer". Aaamolybdenum.com. Archived from the original on 2003-02-09. Retrieved 2012-08-06.
- ^ G. Glemser "Sodium Rhenate (IV)" Handbook of Preparative Inorganic Chemistry, 2nd Ed. Edited by G. Brauer, Academic Press, 1963, NY. Vol. 1. p. 1483.
- ^ Schmidt, Max; Schmidbaur, Hubert (1967). "Trimethylsilyl Perrhenate". Inorganic Syntheses. Vol. 9. pp. 149–151. doi:10.1002/9780470132401.ch40. ISBN 9780470132401.
- ^ a b O. Glemser (1963). "Rhenium". In G. Brauer (ed.). Handbook of Preparative Inorganic Chemistry. Vol. 1 (2nd ed.). Academic Press. pp. 1476–1485.
- ^ Georg Nadler, Hans (2000). "Rhenium and Rhenium Compounds". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a23_199. ISBN 3527306730.
- ^ Nechamkin, H.; Hiskey, C. F.; Moeller, Therald; Shoemaker, C. E. (Jan 1950), Audrieth, Ludwig F. (ed.), "Rhenium(VI) Oxide (Rhenium Trioxide)", Inorganic Syntheses, vol. 3 (1 ed.), Wiley, pp. 186–188, doi:10.1002/9780470132340.ch49, ISBN 978-0-470-13162-6, retrieved 2023-08-26
- ^ O. Glemser; R. Sauer (1963). "Rhenium(VI) Oxide". In G. Brauer (ed.). Handbook of Preparative Inorganic Chemistry, 2nd Ed. Vol. 2. NY, NY: Academic Press. p. 1482.
- ^ Stout, J. W.; Reed, Stanley A. (1954). "The Crystal Structure of MnF2, FeF2, CoF2, NiF2 and ZnF2". J. Am. Chem. Soc. 76 (21): 5279–5281. doi:10.1021/ja01650a005.
- ^ Ayres, D. C.; Hellier, Desmond (1997). Dictionary of Environmentally Important Chemicals. CRC Press. p. 195. ISBN 0-7514-0256-7. Retrieved 2008-06-18.
- ^ "Scacchite".
- ^ "Kempite".
- ^ a b Cepanec, Ivica (2004). Synthesis of Biaryls. Elsevier. p. 104. ISBN 0-08-044412-1. Retrieved 2008-06-18.
- ^ Johnstone, E. V. (May 2014). Binary Technetium Halides (Thesis). University of Nevada, Las Vegas. doi:10.34917/5836118 – via UNLV Theses, Dissertations, Professional Papers, and Capstones.
- ^ a b c d Poineau, Frederic; Johnstone, Erik V.; Czerwinski, Kenneth R.; Sattelberger, Alfred P. (2014). "Recent Advances in Technetium Halide Chemistry". Accounts of Chemical Research. 47 (2): 624–632. doi:10.1021/ar400225b. PMID 24393028.
- ^ Poineau, Frederic; Johnstone, Erik V.; Weck, Philippe F.; Kim, Eunja; Forster, Paul M.; Scott, Brian L.; Sattelberger, Alfred P.; Czerwinski, Kenneth R. (2010). "Synthesis and Structure of Technetium Trichloride". Journal of the American Chemical Society. 132 (45): 15864–5. doi:10.1021/ja105730e. PMID 20977207.
- ^ Schwochau 2000, pp. 7–9
- ^ German, K. E.; Kryutchkov, S. V. (2002). "Polynuclear Technetium Halide Clusters". Russian Journal of Inorganic Chemistry. 47 (4): 578–583. Archived from the original on 2015-12-22.
- ^ Cahiez, Gerard; Duplais, Christophe; Buendia, Julien (2009). "Chemistry of Organomanganese(II) Compounds". Chem. Rev. 109 (3): 1434–1476. doi:10.1021/cr800341a. PMID 19209933.
- ^ Layfield, Richard A. (2008). "Manganese(II): The Black Sheep of the Organometallic Family". Chem. Soc. Rev. 37 (6): 1098–1107. doi:10.1039/b708850g. PMID 18497923.
- ^ Bartholomä, Mark D.; Louie, Anika S.; Valliant, John F.; Zubieta, Jon (2010). "Technetium and Gallium Derived Radiopharmaceuticals: Comparing and Contrasting the Chemistry of Two Important Radiometals for the Molecular Imaging Era". Chemical Reviews. 110 (5): 2903–20. doi:10.1021/cr1000755. PMID 20415476.
- ^ a b c Alberto, Roger (2010). "Organometallic Radiopharmaceuticals". Medicinal Organometallic Chemistry. Topics in Organometallic Chemistry. Vol. 32. pp. 219–246. doi:10.1007/978-3-642-13185-1_9. ISBN 978-3-642-13184-4.
- ^ Hileman, J. C.; Huggins, D. K.; Kaesz, H. D. (1961). "Technetium carbonyl". Journal of the American Chemical Society. 83 (13): 2953–2954. doi:10.1021/ja01474a038.
- ^ Bailey, M. F.; Dahl, Lawrence F. (1965). "The Crystal Structure of Ditechnetium Decacarbonyl". Inorganic Chemistry. 4 (8): 1140–1145. doi:10.1021/ic50030a011.
- ^ Wallach, D. (1962). "Unit cell and space group of technetium carbonyl, Tc2(CO)10". Acta Crystallographica. 15 (10): 1058. Bibcode:1962AcCry..15.1058W. doi:10.1107/S0365110X62002789.
- ^ Schwochau 2000, pp. 286, 328.
- ^ Breimair, Josef; Steimann, Manfred; Wagner, Barbara; Beck, Wolfgang (1990). "Nucleophile Addition von Carbonylmetallaten an kationische Alkin-Komplexe [CpL2M(η2-RC≡CR)]+ (M = Ru, Fe): μ-η1:η1-Alkin-verbrückte Komplexe". Chemische Berichte. 123: 7. doi:10.1002/cber.19901230103.
- ^ Schmidt, Steven P.; Trogler, William C.; Basolo, Fred (1990). "Pentacarbonylrhenium Halides". Inorganic Syntheses. Vol. 28. pp. 154–159. doi:10.1002/9780470132593.ch42. ISBN 978-0-470-13259-3.
- ^ Michael A. Urbancic; John R. Shapley (1990). "Pentacarbonylhydridorhenium". Inorganic Syntheses. Vol. 28. pp. 165–168. doi:10.1002/9780470132593.ch43. ISBN 978-0-470-13259-3.
- ^ Hudson, A. (2002) “Methyltrioxorhenium” in Encyclopedia of Reagents for Organic Synthesis. John Wiley & Sons: New York, ISBN 9780470842898, doi:10.1002/047084289X.
- ^ German, Konstantin E.; Fedoseev, Alexander M.; Grigoriev, Mikhail S.; Kirakosyan, Gayane A.; Dumas, Thomas; Den Auwer, Christophe; Moisy, Philippe; Lawler, Keith V.; Forster, Paul M.; Poineau, Frederic (2021-09-24). "A 70-Year-Old Mystery in Technetium Chemistry Explained by the New Technetium Polyoxometalate [H 7 O 3 ] 4 [Tc 20 O 68 ] ⋅ 4H 2 O". Chemistry – A European Journal. 27 (54): 13624–13631. doi:10.1002/chem.202102035. ISSN 0947-6539. PMID 34245056.
- ^ Volkov, Mikhail A.; Novikov, Anton P.; Borisova, Nataliya E.; Grigoriev, Mikhail S.; German, Konstantin E. (2023-08-21). "Intramolecular Re···O Nonvalent Interactions as a Stabilizer of the Polyoxorhenate(VII)". Inorganic Chemistry. 62 (33): 13485–13494. doi:10.1021/acs.inorgchem.3c01863. ISSN 0020-1669. PMID 37599582.
- ^ Chalmin, E.; Vignaud, C.; Salomon, H.; Farges, F.; Susini, J.; Menu, M. (2006). "Minerals discovered in paleolithic black pigments by transmission electron microscopy and micro-X-ray absorption near-edge structure" (PDF). Applied Physics A. 83 (12): 213–218. Bibcode:2006ApPhA..83..213C. doi:10.1007/s00339-006-3510-7. hdl:2268/67458. S2CID 9221234.
- ^ Sayre, E. V.; Smith, R. W. (1961). "Compositional Categories of Ancient Glass". Science. 133 (3467): 1824–1826. Bibcode:1961Sci...133.1824S. doi:10.1126/science.133.3467.1824. PMID 17818999. S2CID 25198686.
- ^ Mccray, W. Patrick (1998). "Glassmaking in renaissance Italy: The innovation of venetian cristallo". JOM. 50 (5): 14–19. Bibcode:1998JOM....50e..14M. doi:10.1007/s11837-998-0024-0. S2CID 111314824.
- ^ Tilgner, Hans Georg (2000). Forschen Suche und Sucht (in German). Books on Demand. ISBN 978-3-89811-272-7.
- ^ "Rhenium: Statistics and Information". Minerals Information. United States Geological Survey. 2011. Retrieved 2011-05-25.
- ^ Moseley, Henry (1914). "The High-Frequency Spectra of the Elements, Part II". Philosophical Magazine. 27 (160): 703–713. doi:10.1080/14786440408635141. Archived from the original on 2010-01-22. Retrieved 2009-05-14.
- ^ Yoshihara, H. K. (2004). "Discovery of a new element 'nipponiumʼ: re-evaluation of pioneering works of Masataka Ogawa and his son Eijiro Ogawa". Spectrochimica Acta Part B: Atomic Spectroscopy. 59 (8): 1305–1310. Bibcode:2004AcSpB..59.1305Y. doi:10.1016/j.sab.2003.12.027.
- ^ Hisamatsu, Yoji; Egashira, Kazuhiro; Maeno, Yoshiteru (2022). "Ogawa's nipponium and its re-assignment to rhenium". Foundations of Chemistry. 24: 15–57. doi:10.1007/s10698-021-09410-x.
- ^ Öhrström, Lars; Reedijk, Jan (28 November 2016). "Names and symbols of the elements with atomic numbers 113, 115, 117 and 118 (IUPAC Recommendations 2016)" (PDF). Pure Appl. Chem. 88 (12): 1225–1229. doi:10.1515/pac-2016-0501. hdl:1887/47427. S2CID 99429711. Retrieved 22 April 2017.
- ^ Noddack, W.; Tacke, I.; Berg, O. (1925). "Die Ekamangane". Naturwissenschaften. 13 (26): 567–574. Bibcode:1925NW.....13..567.. doi:10.1007/BF01558746. S2CID 32974087.
- ^ Noddack, W.; Noddack, I. (1929). "Die Herstellung von einem Gram Rhenium". Zeitschrift für Anorganische und Allgemeine Chemie (in German). 183 (1): 353–375. doi:10.1002/zaac.19291830126.
- ^ Committee On Technical Aspects Of Critical And Strategic Material, National Research Council (U.S.) (1968). Trends in usage of rhenium: Report. pp. 4–5.
- ^ Savitskiĭ, Evgeniĭ Mikhaĭlovich; Tulkina, Mariia Aronovna; Povarova, Kira Borisovna (1970). Rhenium alloys.
- ^ Heiserman, D. L. (1992). "Element 43: Technetium". Exploring Chemical Elements and their Compounds. New York: TAB Books. p. 164. ISBN 978-0-8306-3018-9.
- ^ Segrè, Emilio (1993). A Mind Always in Motion: The Autobiography of Emilio Segrè. Berkeley, California: University of California Press. pp. 115–118. ISBN 978-0520076273.
- ^ Yu; Demin, A.G.; Danilov, N.A.; Flerov, G.N.; Ivanov, M.P.; Iljinov, A.S.; Kolesnikov, N.N.; Markov, B.N.; Plotko, V.M.; Tretyakova, S.P. (1976). "On spontaneous fission of neutron-deficient isotopes of elements". Nuclear Physics A. 273: 505–522. doi:10.1016/0375-9474(76)90607-2.
- ^ a b Barber, R. C.; Greenwood, N. N.; Hrynkiewicz, A. Z.; Jeannin, Y. P.; Lefort, M.; Sakai, M.; Ulehla, I.; Wapstra, A. P.; Wilkinson, D. H. (1993). "Discovery of the transfermium elements. Part II: Introduction to discovery profiles. Part III: Discovery profiles of the transfermium elements". Pure and Applied Chemistry. 65 (8): 1757. doi:10.1351/pac199365081757. S2CID 195819585.
- ^ Münzenberg, G.; Hofmann, S.; Heßberger, F. P.; Reisdorf, W.; Schmidt, K. H.; Schneider, J. H. R.; Armbruster, P.; Sahm, C. C.; Thuma, B. (1981). "Identification of element 107 by α correlation chains". Zeitschrift für Physik A. 300 (1): 107–8. Bibcode:1981ZPhyA.300..107M. doi:10.1007/BF01412623. S2CID 118312056. Retrieved 24 December 2016.
- ^ a b c Emsley 2001, pp. 249–253
- ^ Bhattacharyya, P. K.; Dasgupta, Somnath; Fukuoka, M.; Roy Supriya (1984). "Geochemistry of braunite and associated phases in metamorphosed non-calcareous manganese ores of India". Contributions to Mineralogy and Petrology. 87 (1): 65–71. Bibcode:1984CoMP...87...65B. doi:10.1007/BF00371403. S2CID 129495326.
- ^ a b USGS Mineral Commodity Summaries 2009
- ^ a b Holleman, Arnold F.; Wiberg, Egon; Wiberg, Nils (1985). "Mangan". Lehrbuch der Anorganischen Chemie (in German) (91–100 ed.). Walter de Gruyter. pp. 1110–1117. ISBN 978-3-11-007511-3.
- ^ Cook, Nigel J.; Ciobanu, Cristiana L.; Pring, Allan; Skinner, William; Shimizu, Masaaki; Danyushevsky, Leonid; Saini-Eidukat, Bernhardt; Melcher, Frank (2009). "Trace and minor elements in sphalerite: A LA-ICPMS study". Geochimica et Cosmochimica Acta. 73 (16): 4761–4791. Bibcode:2009GeCoA..73.4761C. doi:10.1016/j.gca.2009.05.045.
- ^ Wang, X; Schröder, HC; Wiens, M; Schlossmacher, U; Müller, WEG (2009). "Manganese/polymetallic nodules: micro-structural characterization of exolithobiontic- and endolithobiontic microbial biofilms by scanning electron microscopy". Micron. 40 (3): 350–358. doi:10.1016/j.micron.2008.10.005. PMID 19027306.
- ^ United Nations Ocean Economics and Technology Office, Technology Branch, United Nations (1978). "Manganese Nodules: Dimensions and Perspectives". Marine Geology. 41 (3–4). Springer: 343. Bibcode:1981MGeol..41..343C. doi:10.1016/0025-3227(81)90092-X. ISBN 978-90-277-0500-6.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "Manganese Mining in South Africa – Overview". MBendi.com. Archived from the original on 5 February 2016. Retrieved 4 January 2014.
- ^ Elliott, R; Coley, K; Mostaghel, S; Barati, M (2018). "Review of Manganese Processing for Production of TRIP/TWIP Steels, Part 1: Current Practice and Processing Fundamentals". JOM. 70 (5): 680–690. Bibcode:2018JOM....70e.680E. doi:10.1007/s11837-018-2769-4. S2CID 139950857.
- ^ Corathers, L. A.; Machamer, J. F. (2006). "Manganese". Industrial Minerals & Rocks: Commodities, Markets, and Uses (7th ed.). SME. pp. 631–636. ISBN 978-0-87335-233-8.
- ^ Zhang, Wensheng; Cheng, Chu Yong (2007). "Manganese metallurgy review. Part I: Leaching of ores/secondary materials and recovery of electrolytic/chemical manganese dioxide". Hydrometallurgy. 89 (3–4): 137–159. Bibcode:2007HydMe..89..137Z. doi:10.1016/j.hydromet.2007.08.010.
- ^ Chow, Norman; Nacu, Anca; Warkentin, Doug; Aksenov, Igor & Teh, Hoe (2010). "The Recovery of Manganese from low grade resources: bench scale metallurgical test program completed" (PDF). Kemetco Research Inc. Archived from the original (PDF) on 2 February 2012.
- ^ "The CIA secret on the ocean floor". BBC News. 19 February 2018. Retrieved 3 May 2018.
- ^ "Project Azorian: The CIA's Declassified History of the Glomar Explorer". National Security Archive at George Washington University. 12 February 2010. Retrieved 18 September 2013.
- ^ Hein, James R. (January 2016). Encyclopedia of Marine Geosciences - Manganese Nodules. Springer. pp. 408–412. Retrieved 2 February 2021.
- ^ Hoseinpour, Vahid; Ghaemi, Nasser (1 December 2018). "Green synthesis of manganese nanoparticles: Applications and future perspective–A review". Journal of Photochemistry and Photobiology B: Biology. 189: 234–243. Bibcode:2018JPPB..189..234H. doi:10.1016/j.jphotobiol.2018.10.022. PMID 30412855. S2CID 53248245. Retrieved 2 February 2021.
- ^ International Seabed Authority. "Polymetallic Nodules" (PDF). isa.org. International Seabed Authority. Archived from the original (PDF) on 23 October 2021. Retrieved 2 February 2021.
- ^ Oebius, Horst U; Becker, Hermann J; Rolinski, Susanne; Jankowski, Jacek A (January 2001). "Parametrization and evaluation of marine environmental impacts produced by deep-sea manganese nodule mining". Deep Sea Research Part II: Topical Studies in Oceanography. 48 (17–18): 3453–3467. Bibcode:2001DSRII..48.3453O. doi:10.1016/s0967-0645(01)00052-2. ISSN 0967-0645.
- ^ Thompson, Kirsten F.; Miller, Kathryn A.; Currie, Duncan; Johnston, Paul; Santillo, David (2018). "Seabed Mining and Approaches to Governance of the Deep Seabed". Frontiers in Marine Science. 5. doi:10.3389/fmars.2018.00480. hdl:10871/130176. S2CID 54465407.
- ^ a b Emsley 2001, pp. 422–425
- ^ Dixon, P.; Curtis, David B.; Musgrave, John; Roensch, Fred; Roach, Jeff; Rokop, Don (1997). "Analysis of Naturally Produced Technetium and Plutonium in Geologic Materials". Analytical Chemistry. 69 (9): 1692–1699. doi:10.1021/ac961159q. PMID 21639292.
- ^ Curtis, D.; Fabryka-Martin, June; Dixon, Paul; Cramer, Jan (1999). "Nature's uncommon elements: plutonium and technetium". Geochimica et Cosmochimica Acta. 63 (2): 275. Bibcode:1999GeCoA..63..275C. doi:10.1016/S0016-7037(98)00282-8.
- ^ Hammond 2004, p. 4-1.
- ^ Moore, C. E. (1951). "Technetium in the Sun". Science. 114 (2951): 59–61. Bibcode:1951Sci...114...59M. doi:10.1126/science.114.2951.59. PMID 17782983.
- ^ "Rhenium - Element information, properties and uses | Periodic Table". www.rsc.org. Retrieved 2019-12-02.
- ^ Emsley 2001, pp. 358–360.
- ^ a b Rouschias, George (1974). "Recent advances in the chemistry of rhenium". Chemical Reviews. 74 (5): 531. doi:10.1021/cr60291a002.
- ^ Anderson, Steve T. "2005 Minerals Yearbook: Chile" (PDF). United States Geological Survey. Retrieved 2008-10-26.
- ^ Korzhinsky, M. A.; Tkachenko, S. I.; Shmulovich, K. I.; Taran Y. A.; Steinberg, G. S. (2004-05-05). "Discovery of a pure rhenium mineral at Kudriavy volcano". Nature. 369 (6475): 51–52. Bibcode:1994Natur.369...51K. doi:10.1038/369051a0. S2CID 4344624.
- ^ Kremenetsky, A. A.; Chaplygin, I. V. (2010). "Concentration of rhenium and other rare metals in gases of the Kudryavy Volcano (Iturup Island, Kurile Islands)". Doklady Earth Sciences. 430 (1): 114. Bibcode:2010DokES.430..114K. doi:10.1134/S1028334X10010253. S2CID 140632604.
- ^ Tessalina, S.; Yudovskaya, M.; Chaplygin, I.; Birck, J.; Capmas, F. (2008). "Sources of unique rhenium enrichment in fumaroles and sulphides at Kudryavy volcano". Geochimica et Cosmochimica Acta. 72 (3): 889. Bibcode:2008GeCoA..72..889T. doi:10.1016/j.gca.2007.11.015.
- ^ "The Mineral Rheniite". Amethyst Galleries.
- ^ John, D. A.; Taylor, R. D. (2016). "Chapter 7: By-Products of Porphyry Copper and Molybdenum Deposits". In Philip L. Verplanck and Murray W. Hitzman (ed.). Rare earth and critical elements in ore deposits. Vol. 18. pp. 137–164. doi:10.5382/Rev.18.07.
- ^ Magyar, Michael J. (January 2012). "Rhenium" (PDF). Mineral Commodity Summaries. U.S. Geological Survey. Retrieved 2013-09-04.
- ^ "MinorMetal prices". minormetals.com. Archived from the original on 2008-05-15. Retrieved 2008-02-17.
- ^ Harvey, Jan (2008-07-10). "Analysis: Super hot metal rhenium may reach "platinum prices"". Reuters India. Archived from the original on 2009-01-11. Retrieved 2008-10-26.
- ^ Rudenko, A.A.; Troshkina, I.D.; Danileyko, V.V.; Barabanov, O.S.; Vatsura, F.Y. (2021). "Prospects for selective-and-advanced recovery of rhenium from pregnant solutions of in-situ leaching of uranium ores at Dobrovolnoye deposit". Gornye Nauki I Tekhnologii = Mining Science and Technology (Russia). 6 (3): 158–169. doi:10.17073/2500-0632-2021-3-158-169. S2CID 241476783.
- ^ a b c Grice, Kyle (2014). "Recent Studies of Rhenium and Manganese Bipyridine Carbonyl Catalysts for the Electrochemical Reduction of CO2". Advances in Inorganic Chemistry. 66: 163–188. doi:10.1016/B978-0-12-420221-4.00005-6. ISBN 9780124202214.
- ^ Bourrez, Marc (2011). "[Mn(bipyridyl)(CO)3Br]: an abundant metal carbonyl complex as efficient electrocatalyst for CO2 reduction". Angewandte Chemie International Edition in English. 50 (42): 9903–9906. doi:10.1002/anie.201103616. PMID 21922614.
- ^ a b c Francke, Robert (2018). "Homogeneously Catalyzed Electroreduction of Carbon Dioxide -- Methods, Mechanisms, and Catalysts". Chemical Reviews. 118 (9): 4631–4701. doi:10.1021/acs.chemrev.7b00459. PMID 29319300.
- ^ Laurence Knight (30 May 2015). "The element that can make bones glow". BBC. Retrieved 30 May 2015.
- ^ Guérin B; Tremblay S; Rodrigue S; Rousseau JA; et al. (2010). "Cyclotron production of 99mTc: an approach to the medical isotope crisis" (PDF). Journal of Nuclear Medicine. 51 (4): 13N–6N. PMID 20351346.
- ^ Rimshaw, S. J. (1968). Hampel, C. A. (ed.). The Encyclopedia of the Chemical Elements. New York: Reinhold Book Corporation. pp. 689–693.
- ^ Schwochau 2000, p. 414.
- ^ Alberto, Roger; Nadeem, Qaisar (2021). "Chapter 7. 99m Technetium-Based Imaging Agents and Developments in 99Tc Chemistry". Metal Ions in Bio-Imaging Techniques. Springer. pp. 195–238. doi:10.1515/9783110685701-013. S2CID 233684677.
- ^ Schwochau 2000, pp. 12–27.
- ^ Schwochau 2000, p. 87.
- ^ James S. Tulenko; Dean Schoenfeld; David Hintenlang; Carl Crane; Shannon Ridgeway; Jose Santiago; Charles Scheer (2006-11-30). University Research Program in Robotics REPORT (PDF) (Report). University of Florida. doi:10.2172/895620. Retrieved 2007-10-12.
- ^ Schwochau 2000, pp. 87–90.
- ^ a b Emsley 2001, p. 425.
- ^ "Ch. 14 Separation Techniques" (PDF). EPA: 402-b-04-001b-14-final. US Environmental Protection Agency. July 2004. Archived (PDF) from the original on 2014-03-08. Retrieved 2008-08-04.
- ^ a b Schwochau 2000, p. 91.
- ^ Hawecker, Jeannot (1984). "Electrocatalytic Reduction of Carbon Dioxide Mediated by Re(bipy)(CO)3Cl (bipy = 2,2'-bipyridine)". J. Chem. Soc., Chem. Commun.: 328–330. doi:10.1039/C39840000328.
- ^ Sullivan, B. Patrick (1985). "One- and Two-electron Pathways in the Electrocatalytic Reduction of CO2 by fac-Re(bpy)(CO)3Cl (bpy = 2,2'-bipyridine)". J. Chem. Soc., Chem. Commun.: 1414–1416. doi:10.1039/C39850001414.
- ^ Hasan, Heather (2008). Manganese. The Rosen Publishing Group. p. 31. ISBN 978-1-4042-1408-8.
- ^ "Manganese Chemical Background". Metcalf Institute for Marine and Environmental Reporting University of Rhode Island. April 2006. Archived from the original on 28 August 2006. Retrieved 30 April 2008.
- ^ "Risk Assessment Information System Toxicity Summary for Manganese". Oak Ridge National Laboratory. Retrieved 23 April 2008.
- ^ Desmet, G.; Myttenaere, C. (1986). Technetium in the environment. Springer. pp. 392–395. ISBN 978-0-85334-421-6.
- ^ Schwochau 2000, pp. 371–381.
- ^ Schwochau 2000, p. 40.
- ^ Emsley 2001, p. 358-361.
- ^ Haley, Thomas J.; Cartwright, Frank D. (1968). "Pharmacology and toxicology of potassium perrhenate and rhenium trichloride". Journal of Pharmaceutical Sciences. 57 (2): 321–323. doi:10.1002/jps.2600570218. PMID 5641681.
- ^ Erikson, K. M.; Aschner, M. (2019). "Manganese: Its Role in Disease and Health". Essential Metals in Medicine: Therapeutic Use and Toxicity of Metal Ions in the Clinic. Metal Ions in Life Sciences. Vol. 19. De Gruyter. pp. 253–266. doi:10.1515/9783110527872-016. ISBN 978-3-11-052787-2. PMID 30855111. S2CID 73725546.
- ^ Emsley 2001, pp. 249–253.
- ^ Takeda, A. (2003). "Manganese action in brain function". Brain Research Reviews. 41 (1): 79–87. doi:10.1016/S0165-0173(02)00234-5. PMID 12505649. S2CID 1922613.
Bibliography
[edit]- Emsley, John (2001). Nature's Building Blocks: An A-Z Guide to the Elements. Oxford, UK: Oxford University Press. ISBN 978-0-19-850340-8.
- Hammond, C. R. (2004). Lide, David R. (ed.). CRC handbook of chemistry and physics : a ready-reference book of chemical and physical data. Boca Raton : CRC Press. ISBN 978-0-8493-0485-9.
- Schwochau, K. (2000). Technetium: Chemistry and Radiopharmaceutical Applications. Weinheim, Germany: Wiley-VCH. ISBN 978-3-527-29496-1.



